Tin Fluoride, Stannous Fluoride USP Grade Manufacturers, with SDS GHS MSDS Sheet |
Supplier, Manufacturer, Exporter of Stannous Fluoride, Muby Chemicals of Mubychem Group, established in 1976, is the original manufacturers of Specialty Chemicals, Pharmaceutical Excipient, Fragrance Food & Flavor chemicals, Reagent Grade Chemicals, Shale Gas Fracturing Chemicals in India. Mubychem Group has several manufacturing facilities spread across Western India and world wide contacts and toll manufacturers. We are exporting globally to countries like USA, Canada, Europe, UAE, South Africa, Tanzania, Kenya, Egypt, Nigeria, Cameroon, Uganda, Turkey, Mexico, Brazil, Chile, Argentina, Dubai, Korea, Vietnam, Thailand, Malaysia, Indonesia, Australia, China, Germany, France, Italy, Portugal, Bangladesh, etc. The products are offered as per required specifications and in correct shape and size in mm or meshs or microns as specified by the buyer. The participating units have one or more accreditations like FDA - cGMP and GLP approval, ISO-9001 Certified, "REACH" Registered, ISO-14001, ISO/IEC 17025, ISO-22000, FSSC 22000, ISO 45001, Kosher Certified, Halal Certified, HACCP, FSSAI. We offer Commercial Pure & IP BP EP Ph Eur USP NF JP FCC Food Grade Analytical Reagent Grades of Chemicals |
| Bookmark this Web Site -- or -- Email This Page Info to a Colleague or Yourself |
Search our website here:







Stannous Fluoride: CAS Number: 7783-47-3, EINECS EC Number: 231-999-3 , Molecular Formula: SnF2, Molecular Weight: 156.7, HS Code ---**
How big is your requirement or how small
We serve it all.
Specifications, Safety Data Sheet, Manufacturing process details, Wholesale retail buy sell prices, Uses etc available on line in these pages for Stannous Fluoride.
For MSDS Sheet Click
SDS MSDS Sheet of Stannous Fluoride Manufacturers
Stannous Fluoride
Tin Fluoride Pure & USP Grade Suppliers

Tin (II) fluoride SnF2, commercially referred to commercially as stannous fluoride. It is a colorless solid used as an ingredient in toothpastes that are typically more expensive than those that use sodium fluoride. Stannous fluoride converts the calcium mineral apatite into fluorapatite, which makes tooth enamel more resistant to bacteria-generated acid attacks. In toothpastes containing calcium minerals, sodium fluoride becomes ineffective over time, while stannous fluoride remains effective in strengthening tooth enamel.
SnF2 acts as a Lewis acid.
SnF2 is a reducing agent.
Stannous Fluoride USP Grade
SnF2 -- 156.71
Tin fluoride (SnF2) [CAS 7783-47-3]
Stannous Fluoride contains not less than 71.2 percent of stannous tin (Sn ++), and not less than 22.3 percent and not more than 25.5 percent of fluoride (F), calculated on the dried basis.
Identification—
A: To 5 mL of a solution (1 in 100) in a test tube add 2 mL of calcium chloride TS: a fine, white precipitate of calcium fluoride is formed.
B: Mix on a spot plate 2 drops of a solution (1 in 100) with 2 drops of silver nitrate TS: a brown-black precipitate is formed.
C: Add 1 drop of a solution (1 in 100) to 2 drops of mercuric chloride: a white, silky precipitate is formed. On further addition of the solution (1 in 100), a brown-black precipitate is formed.
pH: between 2.8 and 3.5, in a freshly prepared 0.4% solution.
Loss on drying: Dry it at 105C for 4 hours: it loses not more than 0.5% of its weight.
Water-insoluble substances: Transfer about 10 g, accurately weighed, to a 400-mL plastic beaker, add 200 mL of water, and stir with a plastic rod for 3 minutes, or until no more solid dissolves. Filter through a tared filtering crucible, and wash thoroughly, first with ammonium fluoride solution (1 in 100), then with water. [NOTE—Prepare and use the filtering crucible in a well-ventilated hood.] Dry the residue at 105C for 4 hours, cool, and weigh: the weight of the residue does not exceed 0.2%.
Antimony: —
Rhodamine B solution: Dissolve 20 mg of rhodamine B in 200 mL of 0.5 N hydrochloric acid.
Standard preparation: Transfer 55.0 mg of antimony potassium tartrate, accurately weighed, to a 200-mL volumetric flask, dissolve in water, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 500-mL volumetric flask, add 6 N hydrochloric acid to volume, and mix.
Test preparation: Transfer 1.0 g of Stannous Fluoride, accurately weighed, to a 50-mL volumetric flask, add 6 N hydrochloric acid to volume, and mix.
Procedure: Pipet 5 mL each of the Standard preparation and the Test preparation into separate 125-mL separators, add 15 mL of hydrochloric acid and 1 g of ceric sulfate, and allow to stand for 5 minutes, with occasional shaking. Add 500 mg of hydroxylamine hydrochloride, and shake for 1 minute. Pipet 15 mL of isopropyl ether into the mixture, shake for 30 seconds, add 7 mL of water, and mix. Cool in a water bath at room temperature for 10 minutes, shake for 30 seconds, allow the layers to separate, and discard the aqueous phase. Add 20 mL of Rhodamine B solution, shake for 30 seconds, and discard the aqueous layer. Decant the ether layer from the top of the separator, and centrifuge, if necessary, to obtain a clear solution. Concomitantly determine the absorbances of the ether solutions from the Test preparation and the Standard preparation at the wavelength of maximum absorbance at about 550 nm, with a suitable spectrophotometer, using water as the blank: the absorbance of the Test preparation does not exceed that of the Standard preparation (0.005%).
Assay for stannous ion: —
0.1 N Potassium iodide-iodate: In a 1000-mL volumetric flask, dissolve 3.567 g of potassium iodate, previously dried at 110 to constant weight, in 200 mL of oxygen-free water containing 1 g of sodium hydroxide and 10 g of potassium iodide, dilute with oxygen-free water to volume, and mix. Standardize this solution by titrating a solution prepared from an accurately weighed quantity of reagent tin (Sn) and hydrochloric acid. Each mL of 0.1 N Potassium iodide-iodate is equivalent to 5.935 mg of Sn.
Procedure: Transfer about 250 mg of Stannous Fluoride, accurately weighed, to a 500-mL conical flask, and add 300 mL of hot, recently boiled 3 N hydrochloric acid. While passing a stream of an oxygen-free inert gas over the surface of the liquid, swirl the flask to dissolve the Stannous Fluoride, and cool to room temperature. Add 5 mL of potassium iodide, and titrate in an inert atmosphere with 0.1 N Potassium iodide-iodate, adding 3 mL of starch as the endpoint is approached. Each mL of 0.1 N Potassium iodide-iodate is equivalent to 5.935 mg of Sn++.
Assay for fluoride: —
[NOTE—Store all solutions, except Buffer solution, in plastic containers.]
Buffer solution: Dissolve 57 mL of glacial acetic acid, 58 g of sodium chloride, and 4 g of (1,2-cyclohexylenedinitrilo)tetraacetic acid in 500 mL of water. Adjust with 5 N sodium hydroxide to a pH of 5.25 ±0.25, dilute with water to 1000 mL, and mix.
Standard preparations: Dissolve an accurately weighed quantity of USP Sodium Fluoride quantitatively in water to obtain a solution containing 420 Cg per mL. Each mL of this solution (Standard preparation A) contains 190 Cg of fluoride ion (10 2 M). Transfer 25.0 mL of Standard preparation A to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (Standard preparation B) contains 19 Cg of fluoride ion per mL (10 3 M). Transfer 25.0 mL of
Standard preparation B to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (Standard preparation C) contains 1.9 Cg of fluoride ion per mL (10 4 M).
Assay preparation: Transfer to a 250-mL volumetric flask about 100 mg of Stannous Fluoride, accurately weighed. Add 50 mL of water, mix vigorously for 5 minutes, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, dilute with water to volume, and mix.
Procedure: Pipet 20 mL of each Standard preparation and of the Assay preparation into separate plastic beakers each containing a plastic-coated stirring bar. Pipet 20 mL of Buffer solution into each beaker. Concomitantly measure the potentials, in mV, of the solutions from the Standard preparations and of the solution from the Assay preparation, with a pH meter capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride-specific ion-indicating electrode and a calomel reference electrode. [NOTE—When taking measurements, immerse the electrodes in the solution, stir on a magnetic stirrer having an insulated top until equilibrium is attained (1 to 2 minutes), and record the potential. Rinse and dry the electrodes between measurements, taking care to avoid damaging the crystal of the specific-ion electrode.] Plot the logarithms of the fluoride-ion concentrations, in Cg per mL, of the Standard preparations versus potential, in mV. From the measured potential of the Assay preparation and the standard reponse line, determine the concentration, C, in Cg per mL, of fluoride ion in the Assay preparation: Calculate the percentage of fluoride (F) in the portion of Stannous Fluoride taken by the formula:
125C / W
in which C is the determined concentration of fluoride, in Cg per mL, in the Assay preparation, and W is the weight, in mg, of Stannous Fluoride taken.
For Original Monographs of IP Indian Pharmacopoeia BP British Pharmacopoeia USP US Pharmacopoeia FCC Food Grade product, please check with the respective web-pages or books.
We manufacture and supply as under:
Strontium Acetate Carbonate Fluoride Nitrate Sulfate.
Manufacturers:
MUBY CHEMICALS
Ambernath Mumbai, Ankleshwar Gujarat, India
TEL: (OFFICE) +912223770100, +912223726950
Current Date Time in India GMT+5:30
e-mail: info@mubychem.com
USA, Canada, Mexico and other American
neighbouring buyers may
e-mail: us@mubychem.com
Call toll-free 1-877-682-9243 (1-877-MUBYCHEM)

Copyright and Usual Disclaimer is Applicable.
Last
20 December, 2024




Exporters to USA Canada UAE Europe South Africa Tanzania Kenya Uganda Egypt Nigeria Turkey Mexico Brazil Argentina Chile Dubai etc.
Global or International Suppliers, Exporters, Importers, Manufacturers
I shall pass through this world, but once. If therefore, there is any good that I can do, or if there is any favor that I can show to a fellow human being, let me do it now. Let me not defer or neglect it. For I shall not tread this way again
Stannous Fluoride SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 20-Nov-20
1. Product Identification
Product Name & Other Names: Stannous Fluoride or Tin Fluoride.
CAS No.: 7783-47-3
EINECS EC-No.: 231-999-3
Molecular Weight: 156.7
Chemical Formula: SnF2
Relevant uses and uses advised against (if any): Industrial Manufacturing.
Suppliers: As per letterhead.
2. Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 4) H302
Skin irritation (Category 2) H315
Serious eye damage (Category 1) H318
Labeling according GHS & Regulation (EC) No 1272/2008
GHS Label Elements 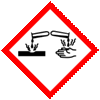 Corrosive |
Signal Words: Danger
Hazard statements:
H302: Harmful if swallowed.
H315: Causes skin irritation.
H318: Causes serious eye damage.
Precautionary statements:
P262: Do not get in eyes, on skin, or on clothing.
P264: Wash skin thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P330: Rinse mouth
P301+P312: IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
P302+P352: IF ON SKIN: Wash with soap and water.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P314: Get Medical advice/attention if you feel unwell.
P330: Rinse mouth.
P332+P313: If skin irritation occurs: Get medical advice/attention.
P362: Take off contaminated clothing and wash before reuse.
P501: Dispose of contents/ container to an approved waste disposal plant.
Classification according to EU Directives 67/548/EEC or 1999/45/EC:
Hazard symbol:
C = Corrosive.
Xn = Harmful.
Risk Phrases:
R22 Harmful if swallowed.
R38 Irritating to skin.
R41 Risk of serious damage to eyes.
3. Composition/Information on Ingredients
Product Name & Other Names: Stannous Fluoride or Tin Fluoride.
CAS No.: 7783-47-3
EINECS EC-No.: 231-999-3
4. First Aid Measures
Always seek medical attention after first aid measures are provided.
Inhalation: Remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.
Ingestion: Never give anything by mouth to an unconscious person. Get medical attention.
Skin Contact: Wipe off excess material from skin then immediately flush skin with plenty of water for at least 15 minutes. Remove contaminated clothing and shoes. Get medical attention. Wash clothing before reuse. Thoroughly clean shoes before reuse.
Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids occasionally. Get medical attention immediately.
5. Fire Fighting Measures
Flammability of the Product: Non-flammable.
Products of Combustion: Hydrogen fluoride gas, Tin oxides.
Fire: Not considered to be a fire hazard.
Fire Extinguishing Media: Use water spray, alcohol-resistant foam, dry chemical, or carbon dioxide. Use means suitable for extinguishing surrounding fire.
Special Information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes. Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used. Uninvolved persons should evacuate to a safe place. On decomposition it may emit hydrogen fluoride. Containers may explode on heating.
6. Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Restrict unprotected personnel from the area. Prevent any contact with hot surfaces. Do not approach facing the wind. Do not touch the spilled material.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage:
Small Spill: Avoid dust formation. Avoid breathing dust. Ensure adequate ventilation. Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements.
Large Spill: Corrosive solid. Avoid touching the spilled material. Do not inhale dust, vapors, mist, or gas. Avoid dust formation. Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate as per law.
7. Handling and Storage
Precautions for safe handling: Do not ingest. Do not breathe dust. Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. In case of insufficient ventilation, wear suitable respiratory equipment. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Prevent any contact with hot surfaces.
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents, metals & acids.
8. Exposure Controls/Personal Protection
Airborne Exposure Limits:
USA ACGIH ACGIH TWA (mg/m³): 2 mg/m³
USA OSHA OSHA PEL (TWA) (mg/m³): 2 mg/m³
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible. Local exhaust ventilation is generally preferred because it can control the emissions of the contaminant at its source, preventing dispersion of it into the general work area.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn. For emergencies or instances where the exposure levels are not known, use a full-face positive-pressure, air-supplied respirator.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Dust deposits on floors and other surfaces may pick up moisture and cause the surfaces to become slippery and present safety hazards. Handle in accordance with good industrial hygiene and safety practice. Wash hands after handling.
9. Physical and Chemical Properties
Appearance: Stannous fluoride is white powder.
Odor: It is odorless.
Odor threshold: Not available.
pH: Not available.
Relative density: around 4.57
Boiling Point: 850C - lit.
Melting Point: 215C.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility: It soluble in water.
Viscosity: Not available.
Molecular Weight: 156.7
Chemical Formula: SnF2
10. Stability and Reactivity
Stability: Stable under ordinary conditions of use and storage.
Hazardous Decomposition Products: It emits toxic fluorine or hydrogen fluoride fumes when heated to decomposition.
Hazardous Polymerization: Will not occur.
Incompatibilities: Strong oxidizing agents, metals & acids. Also incompatible with potassium, Bromine trifluoride, Hydrazine, Ethylene oxide, Metals, organic nitrates sodium, hydrazine, nitromethane, acetylene, sodium hypobromite, alcohols. Halogens.
Conditions to Avoid: Incompatibles and moisture.
11. Toxicological Information
Toxicity data
LD50 Oral - Rat - 360 mg/kg
LD50 Dermal - Rat - > 2,000 mg/kg
Carcinogenic Effects: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC, ACGIH, NTP, OSHA.
Mutagenic Effects: Not available.
Teratogenic Effects: Not available.
Developmental Toxicity: Not available.
12. Ecological Information
Toxicity to fish: Not available
Results of PBT and vPvB assessment: This substance/mixture contains no components considered to be either persistent, bioaccumulative and toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
13. Disposal Considerations
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility. Processing use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state, and local requirements.
14. Transport Information
DOT USA, TDG Canada & ADR/RID Europe:
UN number: 2923 Class: 8 (6.1) Packing group: III
Proper shipping name: Corrosive solid, acidic, inorganic, n.o.s. (Stannous fluoride)
Marine pollutant: No
IMDG/IMO:
UN number: 2923 Class: 8 (6.1) Packing group: III
Proper shipping name: Corrosive solid, acidic, inorganic, n.o.s. (Stannous fluoride)
Marine pollutant: No
IATA:
UN number: 2923 Class: 8 (6.1) Packing group: III
Proper shipping name: Corrosive solid, acidic, inorganic, n.o.s. (Stannous fluoride)
Marine pollutant: No
15. Regulatory Information
USA:
TSCA: Not applicable
SARA 302: No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.
SARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minims) reporting levels established by SARA Title III, Section 313.
SARA 311/312 Hazards: Acute Health Hazard
California Prop. 65 Components: This product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other reproductive harm.
16. Other Information
European Labeling in Accordance with EC Directives:
H302: Harmful if swallowed.
H315: Causes skin irritation.
H318: Causes serious eye damage.
Hazard symbol:
C = Corrosive.
Xi = Irritant.
Risk Phrases:
R22: Harmful if swallowed.
R38 Irritating to skin.
R41 Risk of serious damage to eyes.
DISCLAIMER: The information and recommendations set forth herein are presented in good faith and believed correct as of the date hereof. It is compiled from various sources and it is not necessarily all inclusive nor fully adequate in every circumstance. In addition, these suggestions should not be confused with nor followed in violation of applicable laws, regulations, rules or insurance requirements applicable. This MSDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.















